Abstract
Background: Belantamab mafodotin (belamaf) is a first-in-class antibody-drug conjugate (ADC) targeting B-cell maturation antigen (BCMA). Belamaf has shown clinically meaningful activity as a single agent in relapse/refractory multiple myeloma (RRMM). The Algonquin study demonstrated that the anti-myeloma activities of belamaf are significantly increased by the immunomodulatory drug pomalidomide (POM). The Algonquin study is a two part study designed to evaluate the safety and efficacy of different doses and schedules of belamaf in combination with POM and dexamethasone (DEX) (Bela-Pd) in patients (pts) with RRMM. Here we report updated safety and efficacy data for the subgroup of RRMM pts exposed or refractory to lenalidomide (LEN), a proteasome inhibitor (PI) and an anti-CD38 agent (triple class exposed).
Methods: Eligibility criteria included > 1 prior lines of treatment (LoT), LEN and PI exposure and refractoriness to the last LoT. POM was administered at 4 mg days 21/28 days, DEX 40 mg (20 mg age > 75 years) weekly in conjunction with IV belamaf administered as a SINGLE dose of 1.92 mg/kg Q4W or 2.5 mg/kg Q4W, Q8W or Q12W, or SPLIT equally (2.5 or 3.4 mg/kg) on days 1 and 8 Q4W. Responses were assessed by International Myeloma Working Group (IMWG) criteria and adverse events (AEs) were graded by CTCAE criteria except for corneal findings which were also graded by the pre-specified keratopathy and visual acuity (KVA) scale. In Part 1 up to 12 pts were enrolled in each cohort to inform the recommended part 2 dose (RP2D). In part 2, 23 patients will be enrolled to provide 35 patients (inclusive of the 12 treated at the RP2D of 2.5 mg/kg Q8W in Part 1) for overall response determination.
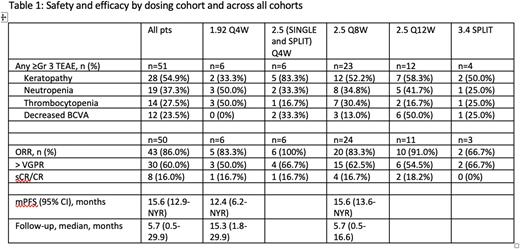
Results: At data cut-off (April 11, 2022), 54 triple class exposed pts had been enrolled in the following dose levels and schedules: 1.92 mg/kg Q4W (n=6), 2.5 mg/kg (SINGLE and SPLIT) Q4W (n=6), 2.5 mg/kg Q8W (n=26), 2.5 mg/kg Q12W (n=12), and 3.4 mg/kg SPLIT (n=4). The median age was 67.5 years (range 36-85) and median prior LoT was 3 (range 2-5). Prior therapies (exposed/refractory) included stem cell transplant (61.1%), PI (100%/79.6%), LEN (100%/92.6%), anti-CD38 (100%/100%) and 72.2% triple class refractory. The most frequent grade 1-2 AEs reported in all cohorts were keratopathy (an ophthalmologic finding) (68.6%), decreased best-corrected visual acuity (BCVA) (60.8%), fatigue (51.0%), thrombocytopenia (35.3%), and blurred vision (33.3%). Grade 3/4 AEs reported in >20% of pts across all cohorts, were keratopathy (54.9%), neutropenia (37.3%), thrombocytopenia (27.5%), and decreased BCVA (23.5%). Three patients discontinued treatment due to AEs (one G4 decreased visual acuity, one G3 elevated ALT, and one due to myelodysplastic syndrome) and three grade 5 AEs were observed (one from myeloma progression, one from lung infection, and one from myelodysplastic syndrome).
At data cut-off, 50/54 patients were evaluable for response. Across all cohorts the ORR was 86.0% (60.0% > VGPR). Responses by dosing cohort are summarized in Table 1. At a median follow-up of 5.7 months, the median PFS (n=49 at a data-cut of March 28, 2022) was 15.6 months (95% CI: 12.9, NYR; across all cohorts).
Conclusions: Patients who are triple class exposed/refractory remain challenging to treat and require therapies with novel mechanisms of action. In the recent LocoMMotion study, triple class exposed MM patients who prospectively received real-life standard of care (SOC) therapies demonstrated an overall response rate (ORR) of 29.8% and median PFS of 4.6 months. Thus, the combination of Bela-Pd represents an improvement over widely available SOC treatments for this poor prognosis patient population.
Disclosures
Trudel:Sanofi: Honoraria; Forus: Consultancy; Janssen: Honoraria, Research Funding; AstraZeneca: Honoraria; Karyopharm: Honoraria; Takeda: Honoraria; Genentech: Research Funding; Pfizer: Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; GlaxoSmithKline: Consultancy, Honoraria, Research Funding. McCurdy:BMS: Honoraria, Research Funding; Sanofi: Honoraria; Janssen: Honoraria; Takeda: Honoraria; GSK: Honoraria; Forus: Honoraria; Amgen: Honoraria. Sutherland:Karyopharm: Research Funding; GSK: Research Funding; Janssen: Consultancy, Research Funding; Amgen: Consultancy; Celgene: Consultancy; Sanofi: Consultancy. Louzada:Celgene: Honoraria; Amgen: Honoraria; Pfizer: Honoraria; Janssen: Honoraria. Chu:Bristol Myers Squibb: Honoraria; Gilead: Honoraria; Sanofi: Honoraria; Janssen: Honoraria. White:Takeda: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria; Antengene: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Forus: Consultancy, Honoraria; GSK: Consultancy, Honoraria. Mian:Sanofi: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria. Kotb:BMS: Honoraria; Sanofi: Honoraria, Research Funding; Amgen: Honoraria; Janssen: Honoraria; Pfizer: Honoraria; Merck: Honoraria, Research Funding; Karyopharm: Current equity holder in private company; Akcea: Honoraria; Takeda: Honoraria; Celgene: Honoraria. Reece:BMS: Research Funding; Sanofi: Honoraria; Amgen: Consultancy, Honoraria; Karyopharm: Consultancy; Celgene: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Millenium: Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Merck: Research Funding; Otsuka: Research Funding; GSK: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal